Our online GCP trivia quizzes can be adapted to suit your requirements for taking some of the top GCP quizzes. Up to 12 cash back Certificate Course in Clinical Research CCCRA Clinical Research Course-ICH-GCP E6 R2 Clinical Trials Essential Documents Sponsor Investigator PharmaceuticalsRating.

Clinical Research Associate Resume Samples Velvet Jobs
Since the inception of the Clinical Research Centre by Ministry of Health in August 2000 Good Clinical Practice GCP has become an important aspect in the conduct of clinical trials in Malaysia.
. The revision is intended to increase the efficiency and quality of clinical trials with. Good clinical practice provides a framework of principles that aim to ensure the safety of research. Regardless of whether you use ACRP SoCRA or some other organization study the material provided.
GCP certification is a mandatory requirement for researchers who want to participate in clinical trial. Malaysian Guideline for Good Clinical Practice 4th Ed Malaysian Guideline for Good Clinical Practice 4 th Edition Published by. Physicians researchers and healthcare.
Go beyond the certification study guide. The National Committee for Clinical research NCCR has commissioned the update of the Malaysian Guideline for Good Clinical Practice following the revision of International Council for Harmonisation ICH E6 Good Clinical Practice guidance in November 2016. A comprehensive database of GCP quizzes online test your knowledge with GCP quiz questions.
Prepare for the certification test. Good Clinical Practice Examination. Free shipping on qualified orders.
Associates residents research fellows. 830am 430pm GMT 8 DAY 3. Exam Hall A Level 4 Educational Block Faculty of Medicine UKM Jalan Yaacob Latif 56000 Cheras Kuala Lumpur.
National Committee for Clinical Research NCCR National Pharmaceutical Regulatory Agency NPRA-secretariat Lot 36 Jalan Universiti 46200 Petaling Jaya Selangor Malaysia. Good Clinical Practice GCP Good Clinical Practice GCP is a set of guidelines for clinical research done on human subjects. Malaysian Guideline for Good CliniCal PraCtiCetHird edition introdUCtion to MalaYSian GUideline For GCP Good Clinical Practice GCP is an international ethical and scientific quality standard for designing conducting recording and reporting clinical trials that involve the participation of human subjects.
The following resources are provided to help investigators sponsors and contract research organizations who conduct clinical studies. Study with Quizlet and memorize flashcards containing terms like Any individual member of the clinical trial team designated and supervised by the investigator at a trial site to perform critical trial-related procedures andor to make important trial-related decisions eg. Regulatory Aspects of Clinical Trial in Malaysia.
Special Offers Course Types Course. It is of utmost importance. Ad Browse discover thousands of brands.
Pharmacists at Ministry of Health Malaysia MOH who attended. Compliance with Good Clinical Practice GCP provides assurance that the rights safety and well-being of trial subjects are protected and that the results of the clinical trials are credible. Free easy returns on millions of items.
Once you complete the certification study guide from the group giving the certification test study clinical trial information outside your daily routine. At GCP Finding we pride ourselves at continuously learning and keeping up to date with. Breakfast Preparation for exam.
41 out of 554 reviews3 total hours36 lecturesBeginnerCurrent price. GCP serves as the international standard for designing conduc. Study coordinator d.
Good Clinical Practice GCP is an international standards concerning the ethical and scientific quality in designing implementing documenting and reporting of clinical trials involving human subjects. Read customer reviews find best sellers. Examination at IMU Bukit Jalil.
Training good clinical practice research human subject protection informed consent institutional review board IRB sponsor trial study investigator.

Clinical Research Associate Resume Samples Velvet Jobs

Clinical Research Associate Resume Samples Velvet Jobs

Introduction To Clinical Research Ii

Clinical Research Job Interview Questions And Answers 2020
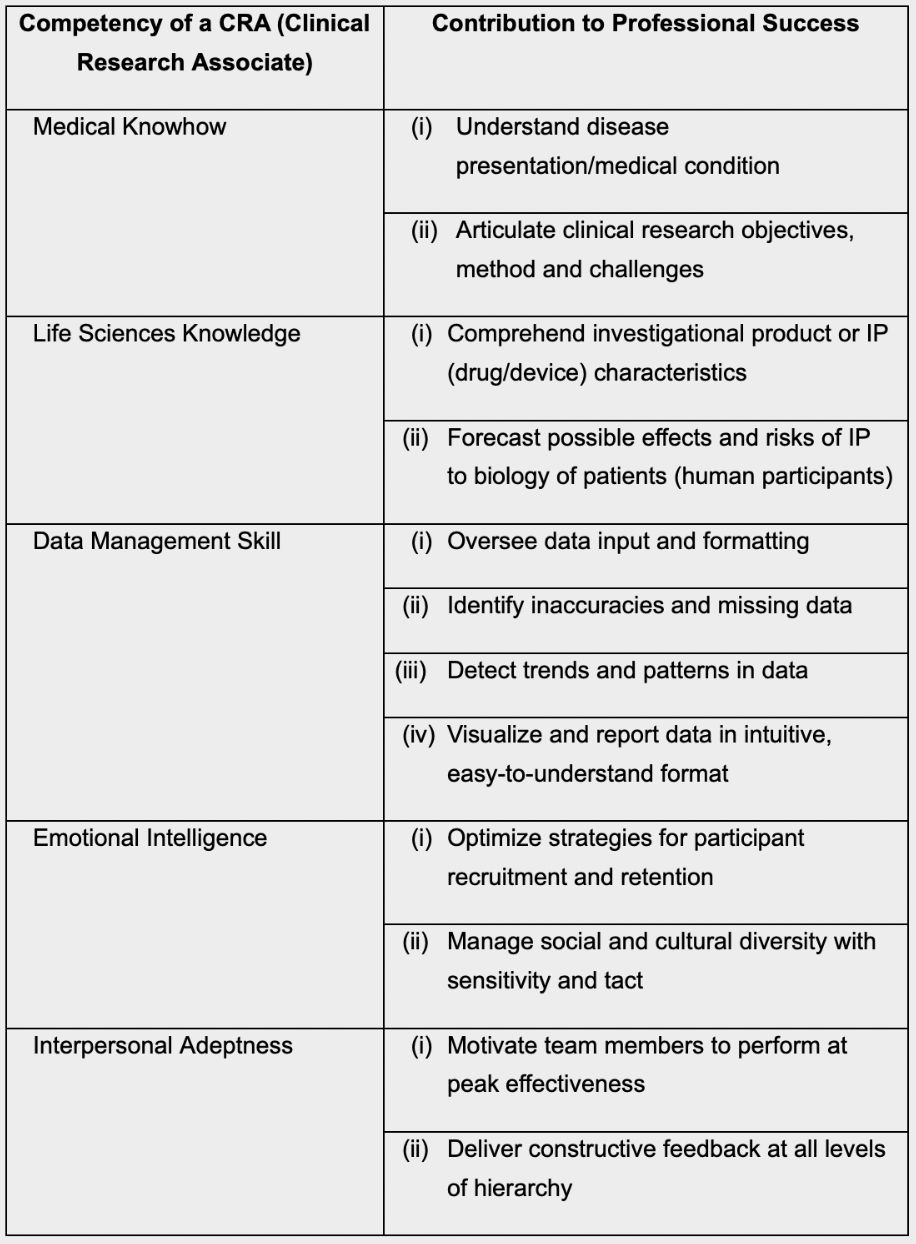
Clinical Research Associate Cra A Complete Guide On How To Become A Clinical Research Associate Clinical Research Certification

Nutrition In Clinical Practice

Pdf Evaluation Of Clinical Practice Guidelines On Fall Prevention And Management For Older Adults A Systematic Review For The Task Force On Global Guidelines For Falls In Older Adults Jama Network
(103).jpg)
Good Clinical Practice Quiz Questions And Answers Proprofs Quiz

Clinical Research Job Interview Questions And Answers 2020
Clinical Research Associate Cra A Complete Guide On How To Become A Clinical Research Associate Clinical Research Certification

Pdf Antisperm Antibody Testing A Comprehensive Review Of Its Role In The Management Of Immunological Male Infertility And Results Of A Global Survey Of Clinical Practices
Clinical Practice Guidelines And Consensus Statements For Antenatal Oral Healthcare An Assessment Of Their Methodological Quality And Content Of Recommendations Plos One

Iq Test Free And No Registration Test Your Intelligence At 123test Com

Pdf Clinical Practice Guidelines For The Management Of Non Specific Low Back Pain In Primary Care An Updated Overview

Clinical Algorithm For Treatment Of Cse In Children Download Scientific Diagram

Introduction To Clinical Research Ii
Clinical Practice Guidelines And Consensus Statements For Antenatal Oral Healthcare An Assessment Of Their Methodological Quality And Content Of Recommendations Plos One

Pdf The Asian Pacific Association For The Study Of The Liver Clinical Practice Guidelines For The Diagnosis And Management Of Metabolic Associated Fatty Liver Disease


